Antimicrobial resistance: time for action on the silent pandemic
Driving innovation in antimicrobials R&D
The World Bank estimates that by 2050, 10 million people per year could die if new antimicrobials are not found[1]. Without effective infection prevention and treatment, medical procedures such as organ transplantation, chemotherapy, and major surgery will become too dangerous to perform. While COVID-19 caught the research and health community off guard, the foreseeable impact of AMR drives us all to be proactive. It's crucial to address this issue before it escalates into a crisis that overshadows even the current challenges we face.
Let's find the solutions
Despite the huge societal costs of AMR and the urgent need for antimicrobials, currently there is no viable market for new antimicrobials and pipelines are sparse.
What are the challenges of antimicrobials R&D?
Creating new antimicrobials is a tough and uncertain process. In other disease areas, investors and developers can depend on the market and incentives to encourage new drug development. But it's not the same for antimicrobials. They have to be used sparingly to remain effective. In practice, this means they won't make enough returns to trigger investment in future research.
Building a robust pipeline for future antimicrobials requires bold actions that recognise and reward successful innovation. This recognition must be substantial enough to give companies confidence to start reinvesting in antimicrobial research and development.
Time to rethink innovation incentives to tackle AMR
The biopharmaceutical industry is engaging in a wide range of activities to promote antimicrobial stewardship to slow the emergence of resistance, prolong the effectiveness of antimicrobials, and improve patient outcomes. In addition, the Innovative Medicines Initiative (IMI) has delivered significant investment and built valuable collaborations in AMR research.
The AMR Action Fund, a ground-breaking partnership involving over 20 leading biopharmaceutical companies, was introduced in 2020 to invest nearly $1 billion to enhance and expedite antibiotic development. The Fund’s ambition is to bring between two and four new antibiotics to patients by 2030. While the Fund offers crucial short-term support, it functions primarily as a bridging mechanism. Its mission acknowledges that further policy reforms are required to ensure a sustainable antimicrobial ecosystem.
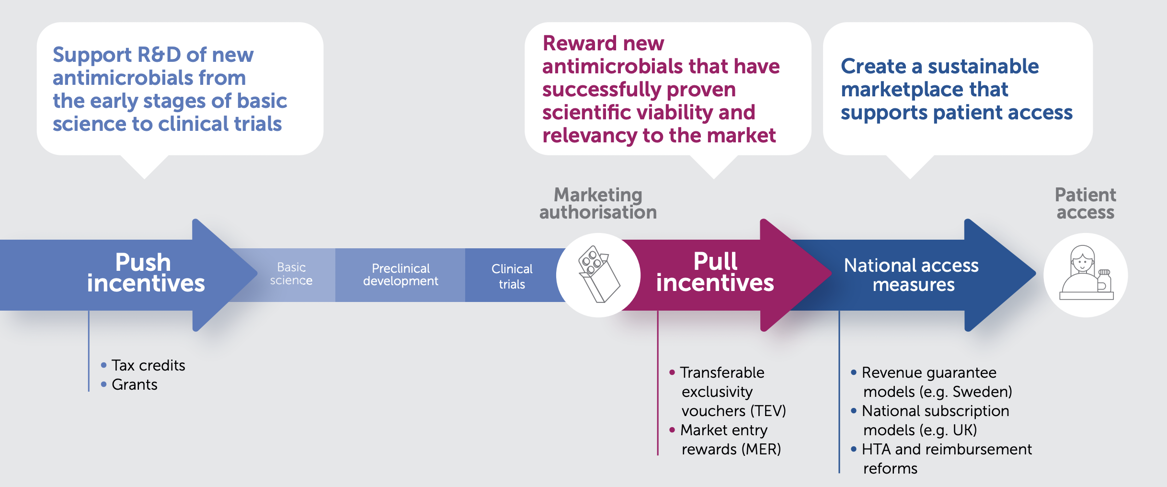
There is widespread agreement on the need for both push and pull incentives to address the lack of R&D of new antimicrobials. Push and pull incentives are complementary, as they seek to address different barriers to the development of new antimicrobials, but ultimately need to work together. Significant progress has been made in the implementation of push incentives. Nonetheless, a deficit in pull incentives persists.
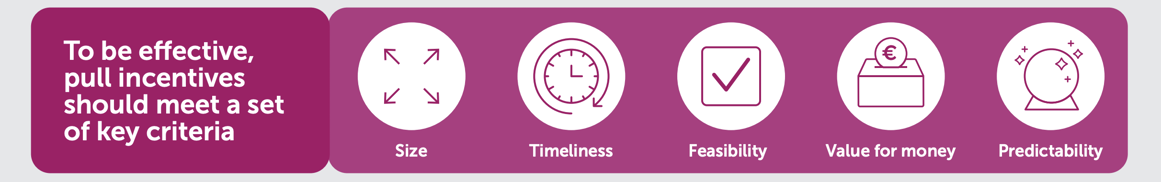
To effectively encourage the development of a sustainable and robust R&D pipeline for novel antimicrobials, while ensuring access to these treatments, it is essential that any pull incentive meets certain criteria:
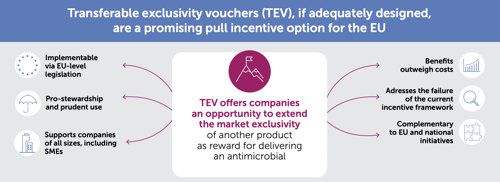
Well-crafted Transferable Exclusivity Vouchers (TEV) have emerged as a promising choice for pull incentives within the EU. TEV can effectively complement other policy-based incentives, such as strengthened value assessment and reimbursement reforms at the national level. Together, these measures synergise to ensure enduring access to vital treatments post-launch, while promoting the sustainability of antimicrobial innovation.
Other resources
- CRA: A forward looking assessment of the cost of introducing TEV get_app
- An assessment of EU Proposals for AMR Incentives against Europe’s fair share get_app
- Assessing the costs of the EC’s proposal for a transferable exclusivity voucher to address AMR get_app
- Novel EU Incentives For Antimicrobials: Time For Ambitious Action get_app
- A framework for assessing the potential net benefits realised through Transferable Exclusivity Extension (TEE) as an incentive for development of novel antimicrobials: FINAL REPORT get_app