HTA & Relative Efficacy Assessment
Assessing the added value of medicines to support access: the benefits of European cooperation
To improve patient access in Europe, EFPIA is calling on HTA bodies to join up when assessing the therapeutic benefit of innovative medicines
Healthcare decision-makers throughout Europe – including national health services, social security institutions, reimbursement agencies, etc. – base their decisions on information and evidence. Health Technology Assessment (HTA) is the tool that decision-makers use to gather and analyse this evidence.
“Meaningful” information on a medicine covers many aspects, from economics to ethics. So HTA uses a broad range of analyses to cover these aspects, including relative efficacy assessment and relative effectiveness assessment. Both look at the medical or therapeutic added value of medicines, compared with alternatives, either in a controlled, trial-based environment (relative efficacy assessment) or in real-life (relative effectiveness assessment).
So What is HTA in a Nutshell?
HTA is a multidisciplinary process that summarises information about the medical, social, economic and ethical issues related to the use of a health technology, in a systematic, transparent, unbiased and robust manner.
What about Relative Efficacy / Relative Effectiveness?
Relative efficacy may be defined as the extent to which an intervention does more good than harm, compared with one or more alternative interventions under ideal circumstances.
Relative effectiveness is the same, but under the usual circumstances of health care practice.
So How Do HTA Agencies Work?
HTA agencies collect and assess information across a wide range of areas on behalf of decision-makers. While the process varies from country to country, this information focuses typically on the medical (relative efficacy and/or relative effectiveness assessment) and economic impact (for example cost-effectiveness assessment or budget impact analysis) of new pharmaceuticals on the individual patient and on the healthcare system as a whole.
Do All Assessments Achieve the Same Results?Surprisingly, HTA agencies reach different conclusions on the medical impact (relative efficacy and/or relative effectiveness assessment) of new pharmaceuticals, even though the data studied is predominantly the same for all markets – such as safety and efficacy data from registration trials. This is because HTA agencies adopt different approaches to rating and interpreting this data. This might apply to trial design, relevant endpoints, appropriateness of defined patient subgroups and treatment comparators.
Equally interesting is the fact that the views of HTA agencies may sometimes be out of step with the outcomes of the European Medicines Agency’s (EMA’s) review of a medicine.
- For companies, this means duplicative administrative work.
- For agencies, this means sometimes inability to conclude on the basis of the evidence provided, because the evidence was generated for other purposes and does not fit national requirements.
- For patients, this means unnecessary trials, potential delays, and access restrictions because of methodological misalignment (rather than the intrinsic properties of products).
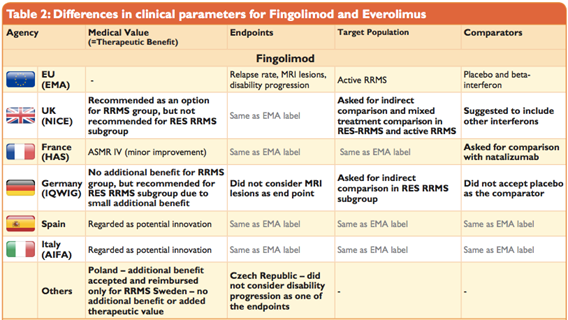
Source: Weber, S., Jain, M., Nallagangula, T. K., Jawla, S., Rai, N., Dev, D., & Cook, N. (2015, November). Heterogeneity in Relative Efficacy Assessments (REA) across European HTA Bodies: Opportunity for Improving Efficiency and Speed of Access to Patients? Poster presented at ISPOR 18th Annual European Congress, 7–11 November 2015, MiCo - Milano Congressi, Milan, Italy
The situation is different when it comes to economics. Economic evaluations rely on locally available data, so different results will be achieved in different countries. This is mainly because European countries enjoy varying economic circumstances and costs of alternative treatments, medical services associated with a condition, including the cost of medical care by healthcare professionals, are likely to diverge.
What Mechanism Do We Have to Address these Hurdles?
The European Network for HTA (EUnetHTA) was set up in 2006, to promote more collaboration and harmonisation in the EU, by linking national HTA agencies, research institutions and health ministries. This allows for an affective exchange of information and lends support to policy decisions by European Member States.
Over time, EUnetHTA has evolved via the mechanism of Joint Action between Member States and the European Commission (Joint Action 1 2010-2012, Joint Action 2 2012-2015, Joint Action 3 2016-2019).
EFPIA has been engaged with EUnetHTA since the start and supports its goal. EFPIA further supports the establishment of a permanent structure of cooperation following the conclusion of Joint Action 3 on HTA in 2020. The European Commission is currently conducting an impact assessment on options post 2020.
Additional resources
- EFPIA position paper on the Commission proposal for a Regulation on HTA get_app
- CRA EFPIA - Learnings from the first three EUnetHTA Joint Action 3 assessments - Final report get_app
- EFPIA response the European Commission HTA consultation (January 2017) get_app
- Assessing the wider benefits of the EU’s proposal on strengthening cooperation on HTA from the industry perspective get_app
How is EUnetHTA Contributing in Practice?
EUnetHTA is addressing divergences by conducting European assessments of the relative efficacy of innovative medicines at the time of launch.
Already, a number of national HTA agencies are working together, together with a range of pharmaceutical manufacturers – all of whom are members of EFPIA.
Pilot projects have shown that through EUnetHTA interested stakeholders can collaborate successfully to produce European reports of relative efficacy at time of launch. However more needs to be done to make sure that national decision-makers accept and recognise these European reports and fully integrate their findings in national access pathways.
EFPIA commissioned a study from Charles River Associates on the current country barriers to adoption of European assessments of relative efficacy at time of launch. In seven of the nine countries analysed, the differences in current methodological approaches for relative efficacy assessment could be easily resolved.
Read more about the key findings of the study
Is this Approach Really Effective?
In 2015, EFPIA commissioned an independent review of the five pilots to review the progress of Joint Action 3. Conducted by Charles River Associates, the review makes clear recommendations on how stronger collaboration in the EU on relative efficacy assessments (REAs) can be achieved during Joint Action 3. These measures will benefit HTA agencies and ultimately patients across the whole of Europe.
We believe that efficient and high quality European REAs of new pharmaceuticals at time of launch are essential. Let’s get on with it! This is the call addressed to Member States by representatives of patients, healthcare professionals, politicians, regulators, HTA agencies, and industry during the World Health Summit in 2015.
Decision-making on pricing, reimbursement and access should remain a national competence. Effectively integrating European REAs in national pricing and reimbursement, though, would issue a strong, positive signal to pharmaceutical companies concerning Europe’s healthcare system priorities.
Ultimately, it will have the potential to reduce time to patient access and strengthen the equity of care throughout Europe.
- Novartis with Midostaurin for the indication of Acute Myeloid Leukaemia. FIMEA and NOMA are EUnetHTA authors of the assessment whilst TLV, ZIN, HAS, NICE, AEMPS, IQWIG are reviewers and SUKL, SU, EOPPY, SESCS are observers.
- Bayer with Regorafenib indicated as monotherapy for the treatment of adult patients with hepatocellular carcinoma (HCC) who have been previously treated with sorafenib treatment. HAS and INFARMED are EUnet HTA authors of the assessment whilst AAZ, SNHTA, FIMEA, LBI, NIPN, AETSA are reviewers and EOF is observer
- Roche with Alecensa indicated as monotherapy for the first-line treatment of adult patients with ALK+ advanced NSCLC. TLV, HVB and AAZ are EUnetHTA authors of the assessment whilst NICE, Regione Veneto, Uniba, AETSA, NIPN are reviewers and MoH Malte is observer.
CRA analysis of the EUnetHTA pilot assessments (2015)
Report on ISPOR session of 10 November 2015
Heterogeneity in relative efficacy assessments (REA) across European HTA bodies: opportunity for improving efficiency and speed of access to patients?
Summary report from the EUnetHTA – EFPIA Expert Meeting (7 October 2015)
Report on World Health Summit session of 12 October 2015
IMS Situational Analyses on Health Technology Assessment (2015)
Making collaborative relative effectiveness assessments relevant: Experience of 5 EUnetHTA pilots across pharmaceuticals and medical devices
HTA Accelerator In-Depth Analysis: Final report
-
EFPIA response to ‘Guidance on outcomes for joint clinical assessments’
We remain optimistic that the flexibility included in the guideline is applied by the assessors in a pragmatic approach. Looking into the future, we hope the second iteration of the guidance will incorporate our recommendations as well as pragmatic learnings from the first years of the JCA process.09.12.24Read Article -
Pharmaceutical innovators are concerned over not enough advice meetings being offered in 2025 for discussing trial designs and evidence generation plans, with serious ramifications on the ability to undergo JCAs
From 12 January 2025 the EU HTA Regulation will apply and the first JCAs will be performed to assess the relative clinical effectiveness of new innovative cancer therapies and cell and gene therapies05.12.24Read Article -
Navigating Certainty and Analytical Complexity: Industry’s Reflections on Ensuring Scientific Rigor and Practical Clarity in Joint Clinical Assessments
The HTA Regulation (HTAR) requires that Joint Clinical Assessments (JCAs) describe the certainty of a health technology’s effects by considering the strengths and limitations of the available evidence.10.11.24Read Article -
Around the world of evidence synthesis (in eighty pages): the methodological and practical guidelines from the HTA Coordination Group. An industry perspective
Application of the European HTA Regulation is around the corner - in only a few months from now, the first products will go through the new European Joint Clinical Assessment (JCA) framework.29.10.24Read Article -
Patient involvement in HTA (Guest blog)
Health Technology Assessments: What can we learn from experiences across Europe?15.05.24Read Article -
Life science industry concerns over the workability of EU HTA: Europe cannot miss out on the opportunity to speed up access to innovative medicines for European patients
We urge the Member States’ representatives to take into account these serious concerns from the innovative life science industry to ensure that the EU HTA procedure can deliver on its intended aims09.04.24Read Article -
Guidance needed to ensure EU Joint Clinical Assessment improves patient access to innovative cancer treatments
New study simulating proposed EU HTA methods for oncology medicines underlines need for guidance on Joint Clinical Assessment process to help faster access for patients28.03.24Read Article -
EU HTA Implementation: Challenges, Commitments and Collective Actions (Guest blog)
There is less than one year to go before EU HTA is either a meaningful contributor to faster access for patients, and the foundation of a stronger and better Europe for life sciences, or becomes an additional hurdle and extra administrative process21.02.24Read Article -
Improving the understanding, acceptance and use of oncology–relevant endpoints in HTA body / payer decision-making
How can we ensure that oncology-relevant endpoints reflect the value of innovation for patients, clinicians, payers and society?12.09.23Read Article -
Joint Statement Pharmaceutical industry concerns over the implementation of the EU HTA Regulation
We call for a constructive dialogue with the European Commission and the newly established Coordination Group, to ensure the HTA Regulation meets its intended objectives26.10.22Read Article -
EFPIA statement on the establishment of the HTA Coordination Group
Europe has a unique opportunity to enact real step change in how it can attract and bring innovation to patients22.06.22Read Article -
How can we make the joint EU HTA more than just a sum of the currently fragmented national work (Guest blog)
During the discussions in Paris, it was evident that the EU HTA regulation will only deliver against its promise, if all stakeholders collaborate during the next coming years.08.03.22Read Article -
EFPIA statement on adoption of Health Technology Assessment Regulation
EFPIA stands ready to play its part to ensure that the future system for joint clinical assessments delivers for patients and that the objective of timely access to innovative medicines is secured14.12.21Read Article -
EU HTA; compromise but at what cost?
Now, more than ever, Europe needs to speak with a coherent voice on clinical evidence in the best interests of patients and our healthcare systems.23.06.21Read Article -
EFPIA statement in response to the Council compromise agreement on the Commission Proposal for a Regulation on Health Technology Assessment
Unfortunately, we regret that the Member States’ compromise risks creating a more inefficient system26.03.21Read Article -
EU-level collaboration on joint clinical assessment of medicines in a post-COVID-19 world
As we get to grips with what the ‘new normal’ looks like, we are also trying to identify the lessons learned from the COVID-19 pandemic. At EFPIA, one of the key insights from the crisis has been the value of collaboration.08.06.20Read Article -
EFPIA statement on European Parliament Plenary on HTA regulation
At today’s Plenary session, the European Parliament adopted the Report on the Commission Proposal for a Regulation on Health Technology Assessment (HTA).03.10.18Read Article -
EFPIA position on proposal for a Regulation of the European Parliament and the Council on HTA and amending Directive 2011/24/EU
This paper outlines EFPIA’s views on the four pillars of EU HTA cooperation included in the Commission proposal08.05.18Read Article -
Meeting with Heads of Agencies/High level representatives and Heads of Europe of Pharmaceutical Companies
European stakeholders of Health Technology Assessments came together to discuss important new developments for further cooperation30.06.17Read Article