Supplementary protection certificates
On 28 May, the European Commission published a legislative proposal amending the SPC Regulation (469/2009) with the objective to introduce a manufacturing waiver aimed at allowing for generic and biosimilar manufacturers to make products during the SPC term, for export purposes to third (non-EU) countries where the IP protection does not exist or has expired.

The Value of IP/SPCs
Pharmaceutical incentives, including Supplementary Protection Certificate (SPCs), are the foundations on which innovation is built. The objective of the SPC was to offset some of the effective patent term lost during the development of a medicine[1]. As such, the SPC Regulation provides companies that research and develop new medicines, the certainty that if a medicine makes it to the market[2], it will be protected from unfair competition for a limited period of time. This is what allows innovative companies to continue to take the risk to invest in the very long, complex, risky and costly process of delivering new medicines to patients, to healthcare systems, and to society and what paves the way for low-cost generics to be used by healthcare systems. 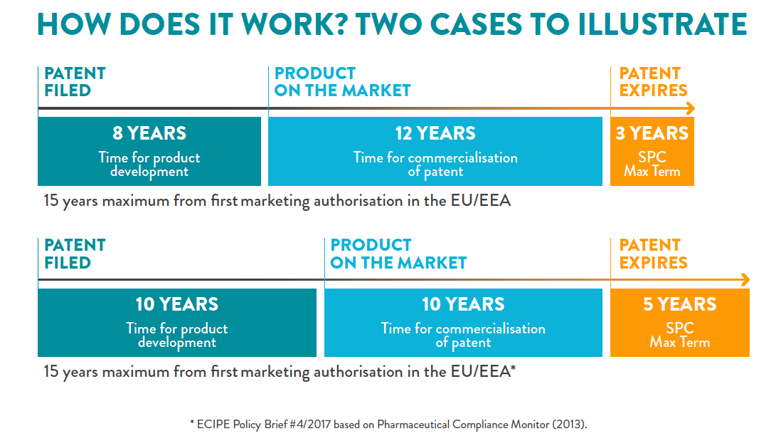
Sustainable healthcare systems benefit from both continuous life-saving innovation and timely access to generics. As such, EFPIA and its membership are fully supportive of immediate generic access after SPC expiry. Today, generic and biosimilar manufacturers make up for 56 percent of the European market and are expected to grow. In addition, they are often available immediately after SPC expiry[3]. Consequently, it should be noted, that the SPC manufacturing waiver will not provide for better generic access as this is the case already today. Looking into the future and the global context, IP incentives remain as important as ever to provide an environment where the pharmaceutical industry can develop solutions to today’s unmet needs and ensure that Europe continues to be an attractive location for R&D investment and industrial development.
Changes to the SPC regulation
On 28 May, the European Commission published a legislative proposal amending the SPC Regulation (469/2009) with the objective to introduce a manufacturing waiver aimed at allowing for generic and biosimilar manufacturers to make products during the SPC term, for export purposes to third (non-EU) countries where the IP protection does not exist or has expired.
“Europe has long benefited from being a global leader in medical innovation based on a framework of IP and incentives that has given investors and companies the assurances they need to invest in the region and grow an industry dedicated to the long, complex and risky process of developing new treatments. In our view, the Commission’s proposal to de-value this crucial framework sends the wrong signal to investors in the global race to attract life science investments. For it not to cause further damage, it is fundamental that it is implemented in a way that continues to support and drive medical innovation” Nathalie Moll, EFPIA’s Director General.
EFPIA is concerned with the Commission’s proposal because:
- The proposal reduces IP rights and sends a signal to the world that Europe is weakening its commitment to intellectual property (IP) incentives and innovation.
- This puts at risk long-term investment in jobs, economic growth and the advancement of patient care in Europe while Europe’s global competitors are taking measures to strengthen their IP framework and attractiveness[4].
Valuing innovation in the context of the proposal
EFPIA believes that it is absolutely vital that the final legislative text provides for clarity and certainty on what the waiver entails and under which conditions it applies to ensure that there is no further erosion of essential innovators’ IP rights. As per the Commission’s proposal[5], the waiver should only apply if the maker (1) has notified its intention to take advantage of the exception, (2) has taken appropriate packaging measures and (3) has informed any of its contractors of the conditions of the exception. EFPIA believes that these safeguards can only be effective if they are clarified and strengthened.
The following are issues that EFPIA has identified:
Transparency & legal certainty – The Commission proposal rightly suggests that there should be ex-ante transparency as regard the generic or biosimilar manufacturer’s intention to rely on the waiver. This entails that, to provide both parties sufficient legal certainty,
- the “making” is for exclusive purpose of export to third countries where protection for the product or medicinal product does not exist or has expired;
- the generic or biosimilar manufacturer must inform the relevant authority of their intention to seek the benefit of the waiver 28 days in advance;
- the right holder should also be notified (and not just the relevant authority);
- the proposed timeframe should be extended to 3 months; and
- the notification should include an exhaustive, rather than indicative, list of markets, to which the generic or biosimilar manufacturer intends to export as well as the earliest date of “making”.
Non-retrospective Implementation – To ensure harmonised implementation across the EU, the waiver should only apply to SPCs applied for on or after the implementation date. This would also preserve the legitimate expectations that any innovator considers when deciding to invest in researching and developing a medicine in a country or region.
Anti-diversion measures – To protect IP rights, it is important that the products, made under the SPC waiver, are not re-imported into the EU or put on the EU market before SPC expiry. To that end, the Commission proposal suggests that a logo is affixed to the outer packaging of the product. This is a good starting point that however can be easily circumvented due to various labelling restrictions in different markets. To ensure an easy, workable and effective solution, EFPIA proposes to rely on the European Medicines Verification System[6] (EMVS), mandated by the Falsified Medicines Directive[7] (FMD). This would be achieved by requiring that the unique identifier (which will be mandatory for any medicine to be dispensed in the EU) cannot be placed on the product intended for export under the manufacturing waiver before the expiry of the SPC in the EU.
EFPIA looks forward to continued engagement with policymakers and stakeholders to ensure that the SPC manufacturing waiver does not undermine the innovation process in Europe that patients, healthcare systems and society are relying on.
Downloads
- Future-proofing EU competitiveness by limiting the negative impact of the SPC manufacturing waiver get_app
- SPC Waiver - Setting the record straight get_app
- Review of CRA’s Report “Assessing the economic impacts of changing exemption provisions during patent and SPC protection in Europe" get_app
- Assessing the impact of proposals for a Supplementary Protection Certificate (SPC) Manufacturing Exemption in the EU get_app
[1] On average, it takes 12-13 years to develop a medicine and to get a Marketing Approval. Effectively, this means that 12-13 out of 20 years of effective patent term, is lost. Source – The Pharmaceutical Industry in Figures 2018
[2] Only 1-2 out of 10 000 molecules make it to the market. Source – The Pharmaceutical Industry in Figures 2018
[3] In the course of the sector inquiry the generic industry maintained that an immediate launch (essentially, on “day one”) after expiry of IP protection can be achieved (e.g. by manufacturing in a country, in which patent protection already expired or never existed and importing/distributing the goods on day one), a fact that is confirmed by the findings of the inquiry, European Commission, Pharmaceutical Sector Inquiry Final Report, 8 July 2009, p. 65. Moreover, where delays in generic launch exists, these relate to a number of reasons from pricing and reimbursement procedures, to market size. When discussing the determinants of generic entry, the CRA report does not list SPCs as a key factor (See CRA, Assessing the economic impacts of changing exemption provisions during patent and SPC protection in Europe, 2017, p. 154 and section 4.7.2.)
[4] State Council of China of 12 April 2018 presided by Premier Mr. Li Keqiang ("Breaking News on New Policies of Innovative Drugs in China", Lexology https://tinyurl.com/y7282oae). — General Oce of the Communist Party of China “The Opinion on [...]Encouraging the Innovation of Drugs and Medical Devices” 8 October 2017. (available at https://tinyurl.com/ybxun92o)
[5] https://ec.europa.eu/docsroom/documents/29462
[6] https://emvo-medicines.eu/mission/emvs/
[7] https://ec.europa.eu/health/human-use/falsified_medicines_en