Innovative Pharmaceutical Manufacturing: Ensuring Access to Quality Medicines
Explore the intricate journey that medicines take from concept to the hands of patients.
The Pharmaceutical Manufacturing Journey
Manufacturing pharmaceuticals is a complex and highly regulated process from the production of Active Pharmaceutical Ingredients (APIs) to the distribution of the final medicinal product. Quality controls are paramount, ensuring that each product meets rigorous standards and adheres to marketing authorizations, regulations, and commitments. Only after fulfilling these requirements can a product be certified and released for wholesale distribution. Our manufacturing facilities operate under strict standards and are subject to regular inspections by competent authorities.
Global Impact of Pharmaceutical Manufacturing
One of the remarkable aspects of pharmaceutical manufacturing is that it ensures the same high-quality product reaches patients worldwide. Local regulatory requirements are integrated into the global manufacturing process, guaranteeing consistency and safety for patients in different parts of the world.
Annual Regulatory GMP/GDP Inspection Survey
Since 2003 EFPIA is being engaged in surveying member companies to map the level of Good Manufacturing Practice (GMP) inspections conducted at each of their manufacturing sites. In recent years, the survey has also incorporated assessments of Good Distribution Practice (GDP) inspections and ISO certifications.
Download the latest Inspection Surveys:
Discover the new Inspection Landscape leaflet.
Innovative Manufacturing: A Boon for Patients and the Economy
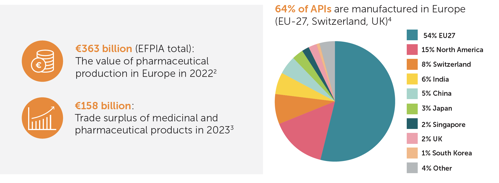
Europe, particularly the EU-27, stands as a prominent hub for the production of innovative medicines and active pharmaceutical ingredients. In 2023, it contributed to a trade surplus of 158 billion euros while continuously supplying patients.
The research-based pharmaceutical industry is committed to investing in cutting-edge manufacturing technologies. These innovations include continuous processing, automation, and modular/mobile manufacturing for various medicines, including vaccines, biologics, and advanced therapies. These advancements not only enhance supply reliability and meet regulatory requirements but also support environmental sustainability and digital transitions.
The changing policy environment may put this contribution at risk.With the revision of the EU pharmaceutical legislation and other legislative files, Europe’s ambition to be a global leader in innovation hangs in the balance.
To remain competitive and drive medical innovation on a global scale, the EU must promote a policy environment that fosters R&D in Europe – the first step to locating advanced manufacturing in the bloc.
This is our Call to Action