2015 Annual Meetings - Big Data: Big Opportunity Luxembourg
03.06.15 - 05.06.15
EFPIA held its 2015 Annual Meetings from 3 to 5 June in Luxembourg. The event provided an unparalleled opportunity to discuss the key issues driving the European pharmaceutical and healthcare agenda as well as network with senior industry and European stakeholders. Below are some highlights of the open sessions.
Enabling better health outcomes through big dataBig Data, Big Opportunity focused on how we develop patient outcomes-driven, sustainable models of healthcare delivery and maximize the potential of the exciting scientific developments.
Against this backdrop, the speakers gave their perspective on how we can use Big Data, in a manner consistent with patients’ rights, to improve the development and delivery of healthcare and help evolve health systems to that end.
EFPIA President Joe Jimenez said: “A focus on outcomes can address many of our current healthcare challenges. Systems are struggling to spend their money where it has the highest impact. A focus on outcomes can help these systems allocate spending where it really makes a difference. By focusing on interventions that really work and moving away from those that don’t, we can improve health and make health systems more financially sustainable.”
In a later meeting with the Prime Minister of Luxembourg, both EFPIA and the Luxembourg Government signalled their desire for collaboration to maximise the potential offered by Big Data in healthcare. The event was followed by a poster session to showcase an important programme within the Innovative Medicines Initiative: “Big Data for Better Outcomes”, which seeks to identify, collect, connect, and analyse big data and use the insights gained to change the way we deliver healthcare.
Pharmaceuticals in the Environment (PiE) – Raising Public AwarenessStefano Soro from SANTE and Helen Clayton from ENV offered presentations on behalf of the European Commission. DG SANTE and DG ENV are leading the development of the PiE strategy.
The public consultation on PiE is expected in the first half of 2016. Both DGs regard PiE as an important emerging topic, where more research is needed. SANTE advocates making policy decisions that are based on sound science.
Bengt Mattson, representing EFPIA, AESGP and EGA, presented the industry associations’ initiative "Eco-Pharmaco-stewardship", which offers solutions that encompass the entire lifecycle of a medicinal product, from manufacturing, through to usage and disposal.
Tiia Metiäinen and a number of partners within the collaboration presented the campaign "Medicines Disposal - it's easier than you think", including the video and website, and an interesting panel discussion took place with representatives from AESGP, EGA, EPSA, FIP and EurEau. The campaign aims to increase citizens’ awareness on how to get rid of their un-used and expired medicines responsibly and in an appropriate manner. Medicines collection schemes vary from country to another. The campaign will be active on Twitter and facebook.
During the PiE workshop, we gathered useful information and tips on how to further develop the campaign: translations/dubbing were recommended as well as initiating the campaign nationally.
General AssemblyWe would like to congratulate Joe Jimenez on his appointment as EFPIA President 2015-2017. Marc de Garidel and Stefan Oschmann were appointed as EFPIA Vice-Presidents 2015-2017.
The EFPIA General Assembly approved the Annual Report 2014, changes to the statutes, EFPIA budget, changes to the EFPIA Governance structure and the priorities for 2015-2016.
Partners in ResearchThe session on Partners in Research explored the collaborative approaches taken by EFPIA, its members and other healthcare companies across a spectrum of health-related sectors and within the framework of IMI2. An intensive debate ensued and we arrived at some concrete and pursuable conclusions.
Speakers settled on the vision that it is only by joining the dots that we will be able to harness the potential of all the enabling technologies on offer: we need urgently the creation of new operational and business models. Going forward, it will be essential to develop a top down and bottom up approach to develop an integrated strategy. As we are fully aware, it is through ongoing and future IMI projects as well as the expertise and authority lent by EFPIA itself that should enable all those involved to catalyse this effort. Moreover, the “big data for better outcomes” concept will serve as an effective umbrella under which to house these efforts.
Finally, there is a need for all partners to think big and project ourselves into the future.
Medicines Adaptive Pathways to Patients (MAPPs)The session sought to flesh out essentially what Medicines Adaptive Pathways to Patients (MAPPs) is.
Great progress has been reported since last year. There have been to date 58 applications to European Medicines Agency’s (EMA’s) pilot in this field, as well as the establishment of an IMI stakeholders platform to coordinate efforts in place. We can report with confidence that most stakeholders are actively engaged in ongoing efforts to clarify and explore the potential of MAPPs.
There remain nevertheless some sticking points. Adaptive pricing and our ability apply it effectively is proving to be a challenge.
Nevertheless, opportunities offered by science and the continual evolution of healthcare systems are driving this change - and solutions will have to be found. Collaborative safe-harbours such as IMI will be instrumental in helping us to achieve this change.
The entire MAPPs session can be viewed here.
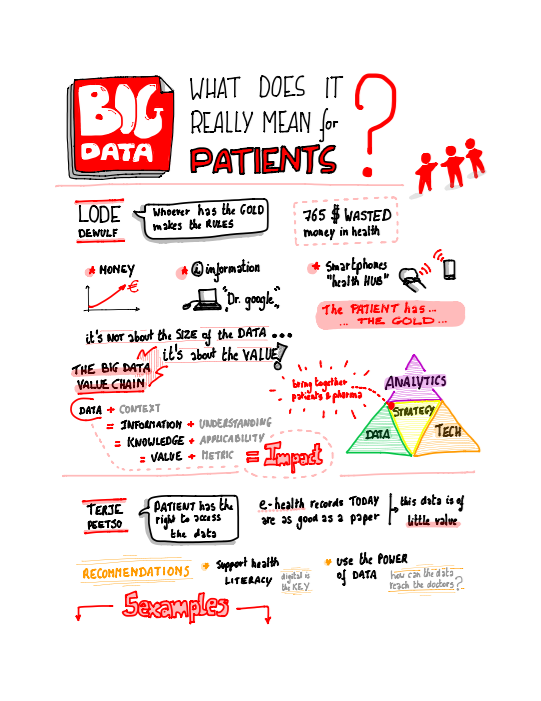
The visual minutes of the "Big data. What does it really mean for patients?" session are available below.
Enabling better health outcomes through big dataBig Data, Big Opportunity focused on how we develop patient outcomes-driven, sustainable models of healthcare delivery and maximize the potential of the exciting scientific developments.
Against this backdrop, the speakers gave their perspective on how we can use Big Data, in a manner consistent with patients’ rights, to improve the development and delivery of healthcare and help evolve health systems to that end.
EFPIA President Joe Jimenez said: “A focus on outcomes can address many of our current healthcare challenges. Systems are struggling to spend their money where it has the highest impact. A focus on outcomes can help these systems allocate spending where it really makes a difference. By focusing on interventions that really work and moving away from those that don’t, we can improve health and make health systems more financially sustainable.”
In a later meeting with the Prime Minister of Luxembourg, both EFPIA and the Luxembourg Government signalled their desire for collaboration to maximise the potential offered by Big Data in healthcare. The event was followed by a poster session to showcase an important programme within the Innovative Medicines Initiative: “Big Data for Better Outcomes”, which seeks to identify, collect, connect, and analyse big data and use the insights gained to change the way we deliver healthcare.
Pharmaceuticals in the Environment (PiE) – Raising Public AwarenessStefano Soro from SANTE and Helen Clayton from ENV offered presentations on behalf of the European Commission. DG SANTE and DG ENV are leading the development of the PiE strategy.
The public consultation on PiE is expected in the first half of 2016. Both DGs regard PiE as an important emerging topic, where more research is needed. SANTE advocates making policy decisions that are based on sound science.
Bengt Mattson, representing EFPIA, AESGP and EGA, presented the industry associations’ initiative "Eco-Pharmaco-stewardship", which offers solutions that encompass the entire lifecycle of a medicinal product, from manufacturing, through to usage and disposal.
Tiia Metiäinen and a number of partners within the collaboration presented the campaign "Medicines Disposal - it's easier than you think", including the video and website, and an interesting panel discussion took place with representatives from AESGP, EGA, EPSA, FIP and EurEau. The campaign aims to increase citizens’ awareness on how to get rid of their un-used and expired medicines responsibly and in an appropriate manner. Medicines collection schemes vary from country to another. The campaign will be active on Twitter and facebook.
During the PiE workshop, we gathered useful information and tips on how to further develop the campaign: translations/dubbing were recommended as well as initiating the campaign nationally.
General AssemblyWe would like to congratulate Joe Jimenez on his appointment as EFPIA President 2015-2017. Marc de Garidel and Stefan Oschmann were appointed as EFPIA Vice-Presidents 2015-2017.
The EFPIA General Assembly approved the Annual Report 2014, changes to the statutes, EFPIA budget, changes to the EFPIA Governance structure and the priorities for 2015-2016.
Partners in ResearchThe session on Partners in Research explored the collaborative approaches taken by EFPIA, its members and other healthcare companies across a spectrum of health-related sectors and within the framework of IMI2. An intensive debate ensued and we arrived at some concrete and pursuable conclusions.
Speakers settled on the vision that it is only by joining the dots that we will be able to harness the potential of all the enabling technologies on offer: we need urgently the creation of new operational and business models. Going forward, it will be essential to develop a top down and bottom up approach to develop an integrated strategy. As we are fully aware, it is through ongoing and future IMI projects as well as the expertise and authority lent by EFPIA itself that should enable all those involved to catalyse this effort. Moreover, the “big data for better outcomes” concept will serve as an effective umbrella under which to house these efforts.
Finally, there is a need for all partners to think big and project ourselves into the future.
Medicines Adaptive Pathways to Patients (MAPPs)The session sought to flesh out essentially what Medicines Adaptive Pathways to Patients (MAPPs) is.
Great progress has been reported since last year. There have been to date 58 applications to European Medicines Agency’s (EMA’s) pilot in this field, as well as the establishment of an IMI stakeholders platform to coordinate efforts in place. We can report with confidence that most stakeholders are actively engaged in ongoing efforts to clarify and explore the potential of MAPPs.
There remain nevertheless some sticking points. Adaptive pricing and our ability apply it effectively is proving to be a challenge.
Nevertheless, opportunities offered by science and the continual evolution of healthcare systems are driving this change - and solutions will have to be found. Collaborative safe-harbours such as IMI will be instrumental in helping us to achieve this change.
The entire MAPPs session can be viewed here.
The visual minutes of the "Big data. What does it really mean for patients?" session are available below.