Getting the world vaccinated against COVID-19
With distribution no longer being a bottleneck, we need to focus all energy on the main remaining barriers to vaccine equity
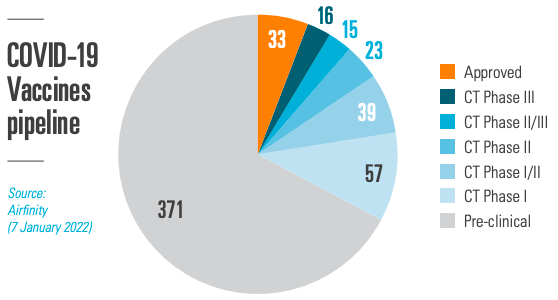
Within 326 days from the WHO declaring COVID-19 a global pandemic, the first vaccine was approved, and, shortly thereafter, administered. Since then, 33 vaccines (5.9% of all vaccines being researched) have received permissions for use by regulatory authorities. Most R&D is therefore still ongoing, also for COVID-19 treatments, where only 1.9% have received regulatory approvals. Scientists could move at such a remarkable pace thanks to decades of prior research, underpinned by a strong IP framework. Preserving the ability to innovate is essential to secure the future R&D pipeline.
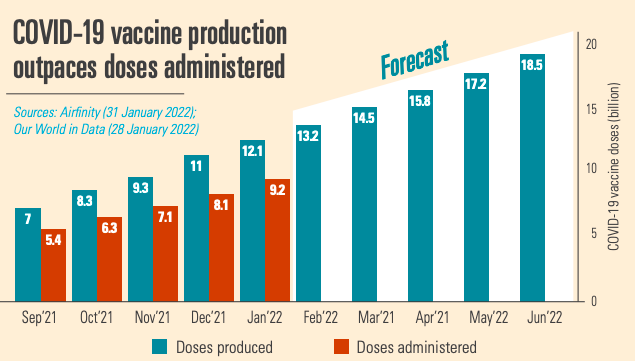
Starting from scratch in 2021, by January 2022 12.1 billion COVID-19 vaccine doses have been produced, 2.9 billion doses more than have been administered. So vaccine production is not the bottleneck. The manufacturing scale up would not have been possible without building new production lines able to produce millions of doses to the highest quality standards, managing global supply chains for hundreds of ingredients, and forging 357 voluntary licensing agreements around the world to increase manufacturing output. Further production expansions are planned, notably in Africa.
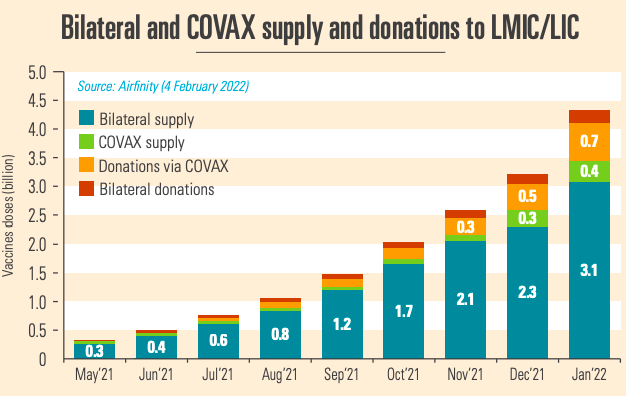
Produced vaccines need to be distributed globally. Trade restrictions have proved to be major bottlenecks to distribution. But in the second half of 2021 exports have increased rapidly, with major vaccine producing countries easing export restrictions on vaccines and supplies. This has led to 4.2 billion vaccines reaching lower middle income countries (LMICs) and low income countries (LICs), with COVAX shipments peaking in December 2021. With distribution no longer being a barrier to vaccine equity either, attention needs to focus on ensuring that distributed vaccines are swiftly deployed.
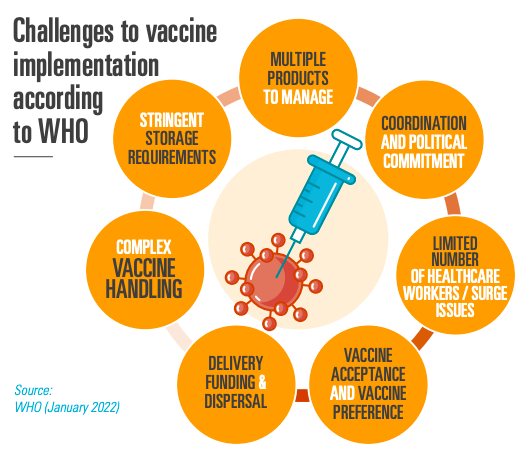
Healthcare system capacities and vaccine hesitancy are key factors that hold back vaccination success, according to the WHO. It is now time to focus efforts on turning ‘vaccines’ into ‘vaccinations’. To this end, ensuring country readiness (including coordination of roll-out campaigns, adequate storage and distribution infrastructure, more trained healthcare workers, and sufficient delivery funding and dispersal) and informing citizens that vaccines are safe, are vital to get vaccines administered. 0.99 bn adults in LMICs and LICs still have not been fully vaccinated.
Step 5: R&D and the emergence of new variants
R&D has given the world safe and effective tools against COVID-19. While effective vaccines and treatments are entering the market as regulatory approvals continue, we have only seen the tip of the iceberg. The approved vaccines and treatments represent only a very small fraction of the R&D pipeline. We cannot let our guard down – with the level of risk of emerging new variants remaining medium to high, continued R&D and testing of efficacy of current vaccines and treatments against Variants of Concern (e.g. Delta, Omicron) remains vital.
Evidence shows that production is not standing in the way of vaccine equity. Also global distribution is decreasingly a bottleneck to access to vaccines, as unused stocks around the world are rising fast. IP has been a key enabler throughout – and continues to be so. It is because of the investment in R&D and a predictable IP system that we increasingly have vaccines and treatments to fight COVID-19. The bottlenecks that hamper success to get vaccines in people’s arms are inadequate healthcare system capacities and vaccine hesitancy. Diverting energy away from these real bottlenecks is misguided at best and disincentivising innovation that we continue to need to fight this pandemic (and future ones) is the wrong way to go. It would be most effective to focus all energy on the main remaining barriers to vaccine equity, while – at the same time – making sure that trade barriers continue to go down.
