How Regulation Can Boost the EU Innovation?
Technology moves fast. An agile and streamlined innovation ecosystem would make Europe more competitive and bring breakthroughs to patients.
We have reached an important moment for Europe’s ambition to be a global leader in innovation. The pharmaceutical industry is a key source of technological advances that improve the lives of patients, as well as a major driver of growth, jobs and creativity in the wider economy.
Regulation plays a central role in the development and use of safe, effective and medicines with impeccable quality. The revision of the current EU regulatory framework for pharmaceuticals is a chance to create a predictable, clear and consistent environment.

If healthcare systems struggle to adapt, innovation will outpace the ability of laws, regulations and healthcare practices to embrace progress. This poses problems for industry, while acting as a brake on patient access to the fruits of scientific progress.
The path forward is to devise pharmaceutical legislation with adequate agility to futureproof it for emerging scientific discoveries. Adaptability is essential. The ability of our system to adapt to the Covid-19 pandemic has shown what is possible. This experience illustrates the value of taking a risk-based approach, leaving the innovator the freedom to operate within a set of agreed boundaries.
It should be noted that alongside the radical innovation powered by breakthrough technologies, routine innovation also continues to address unmet need. For example, changing the formulation of a medication can allow it to be administered as a subcutaneous injection rather than by intravenous infusion – saving time and resources, while offering convenience and quality of life for patients. Well-designed policy instruments must account for all types of innovation.
Predictable, streamlined legislation
An efficient regulatory ecosystem can provide value to patients and the health system but, as all regulation comes with costs for innovators (and for the system), it is important to get the balance right. A business’s ability to innovate is framed by the innovation system in which it is embedded. Complying with multiple layers of governance at EU and national level can add time and costs to the process.
For example, the European system features multiple networks of decision-makers, including medicines regulators, Notified Bodies, and Health Technology Assessment bodies. These stakeholders have different ways of working, diverse needs, and a variety of expectations of innovators. Such complexities can add unpredictability and bureaucracy.
EU-level legislation should aim to simplify and streamline processes, while Member States must strive to avoid complication and contradictions in how rules are implemented. There is a clear need for regulatory convergence between jurisdictions to avoid inefficiencies.
What is needed is a system that is consistent, predictable, and adaptable. It is paramount for the innovators but also for the society, which will bear the cost of less research, less innovation and by that, decline or stagnation in welfare. Unless we get this right, Europe will suffer a decline in its capacity to attract international investment. Reduced global competitiveness would have significant impacts on economic growth and on the fortunes of patients awaiting access to innovative tools and therapies.
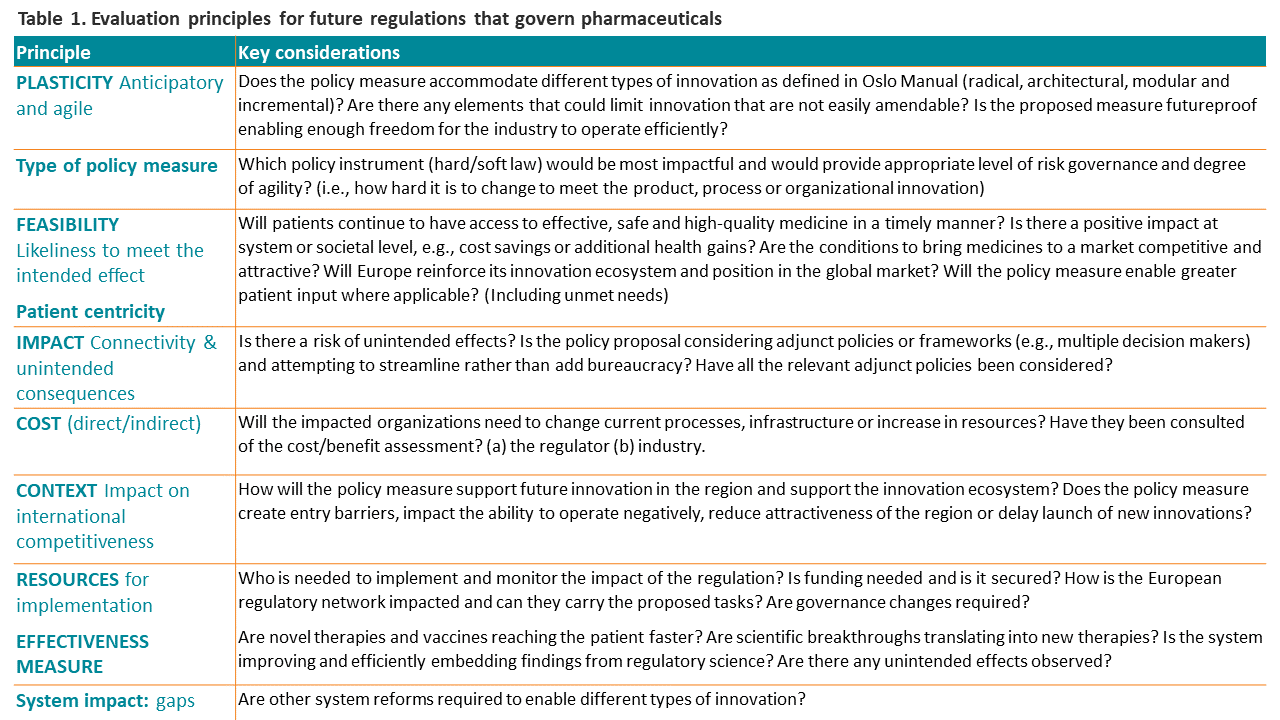
Key factors in designing a regulatory framework
While continuing to rely on the precautionary principle of the pharmaceutical legislation, the EFPIA Innovation Board proposes in addition to the evaluation principles, actions for the European policymakers, to support a more streamlined regulatory system.
- Adaptability: Use guidance documents as soft law tools to enable agile adoption of new tools and methodologies in line with the fast-paced evolution of science and technology.
- Regulatory science: Strengthen further emphasis on regulatory science to inform how the regulatory system is performing and support fit-for-purpose assessment of tools and methodologies.
- Effective patient focus tools: Conduct a thorough cost/benefit assessment of the new regulatory tools to ensure they have an overall positive impact, particularly from the patient perspective, as the ultimate beneficiary but also from the system perspective, to be able to carry the tasks given to the regulator or avoid burdening the regulated industry unless clear benefits are gained.
- Stakeholder engagement: Leverage stakeholder consultations to ensure that the framework actively mitigates negative externalities or unintended effects for every type of innovation or different types of products, which are often unavoidable with regulation.
The beauty of a fast-moving innovative sector is that we cannot know for sure what the future will bring. We can, however, build a system capable of coping with change while consistently applying agreed principles that support safe and timely access to medicines.
There is a lot at stake. Europe must take this opportunity for active dialogue with industry experts on building a fit-for-purpose innovation ecosystem.
NOTE: This blog is based on the research article “Role of innovation in pharmaceutical regulation: a proposal for principles to evaluate EU General Pharmaceutical Legislation from the innovator perspective” by Heikkinen et al, available online as pre-proof before print, in Drug Discovery Today. LINK: https://doi.org/10.1016/j.drudis.2023.103526
Authors: Inkatuuli Heikkinen 1, Sini Eskola 2, Virginia Acha 1, Alan Morrison 1, Chris Walker 3,
Catherine Weil 4, Antoine Bril 5, Max Wegner 6, Thomas Metcalfe 7, Salah-Dine Chibout 8, Magda Chlebus 2 (1 MSD; 2 EFPIA; 3 Amgen; 4 BMS; 5 Servier; 6 Bayer;
7 Roche; 8 Novartis)


