MSD Policy Passport on Pharmaceutical Innovation (Guest blog)
Taking stock of these challenges and the importance for European patients, health systems and economy of a robust research-based pharmaceutical industry, MSD decided to develop a “Policy Passport on Pharmaceutical Innovation”. This document is intended to provide policy makers with a “roadmap” of the drivers and critical policies that support pharmaceutical innovation. By means of reviewing the various issues under discussion across the EU regarding pharmaceutical policy, we wanted to highlight the critical role played by government policies in creating the right conditions for pharmaceutical companies to invest over €36.5 billion in research and development in the EU alone in 2018. [7]
However, the long and risky path pharmaceutical companies embark on to produce innovative medicines remains poorly understood.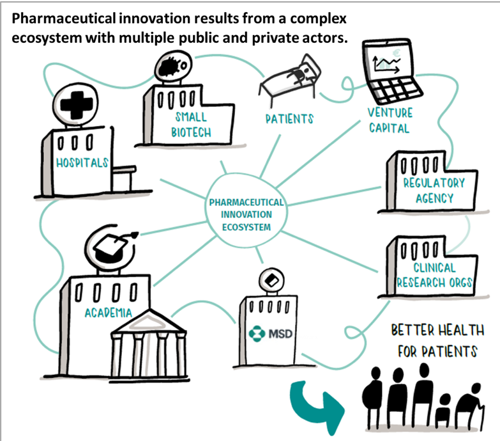
Medicines Don’t Grow on Trees
Clearly this is a complex industry, which is at the heart of a complex ecosystem mixing public and private actors. The breadth of activities taken on by pharmaceutical companies is staggering. Key elements include research in fundamental science to understanding biological mechanisms, screening of large compound libraries to find potential targets, and deploying large and complex clinical trials to demonstrate the safety and efficacy of our products. This explains why the pharmaceutical and biotechnology sector amounts to 18.9% of total business R&D expenditure worldwide. [3] It is also the sector with the highest ratio of R&D investments to sales: In 2016 and 2017, MSD alone invested over $10 billion per year, representing about 25% of our sales. [4]
And all this is only made possible thanks to government policies that support innovation.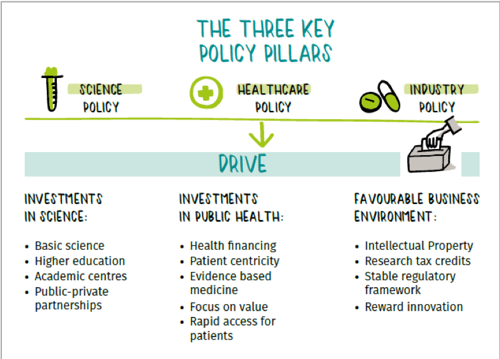
Government Policies Matter
This level of complexity, and even fragmentation throughout the research and development of new medicines, results in a clear challenge for policy makers who regulate our industry. The first question is where should pharmaceutical policy be decided? Is it a public health question, or an industrial policy issue? In fact, governments play a key role through three main pillars: Investment in fundamental research, sustained efforts to improve population health through sustainable healthcare systems, and the implementation of robust industrial policies, such as intellectual property rights. How these policies are shaped, and the decisions taken by policy makers and regulators, can either accelerate or deter R&D investments. This can make the difference between providing a new medicine or no treatment at all for patients.
One of the best illustrations of the role played by policy makers is the implementation of the Orphan Medicines Regulation (EC No141/2000). This Regulation was intended to increase R&D efforts and investments towards rare diseases. Before the EU Orphan Regulation only 8 medicines were licensed for treating rare diseases in Europe. Today, over 160 new treatments have been authorised by the European Medicines Agency.[5]
Another illustration, but in reverse, can be taken from the increased concern over the availability of new antimicrobials in order to address the rise of antimicrobial resistance. Every year at least 25,000 people in the EU alone die from infections caused by resistant bacteria. Unless action is taken, we could revert to a world where simple infections are no longer treatable. The EU and Member State governments can address these barriers and revitalise R&D in this area through a suite of push and pull incentives to support antimicrobial innovation and appropriate use. MSD is among the few large companies remaining fully invested in developing new antimicrobials, and has proposed a Global Action Plan to address antimicrobial resistance and unlock increased R&D investments in this field. [6]
Untangling a Web of Health, Science, and Industrial Policies
Taking stock of these challenges and the importance for European patients, health systems and economy of a robust research-based pharmaceutical industry, MSD decided to develop a “Policy Passport on Pharmaceutical Innovation”. This document is intended to provide policy makers with a “roadmap” of the drivers and critical policies that support pharmaceutical innovation. By means of reviewing the various issues under discussion across the EU regarding pharmaceutical policy, we wanted to highlight the critical role played by government policies in creating the right conditions for pharmaceutical companies to invest over €36.5 billion in research and development in the EU alone in 2018. [7]
Acting as a Partner for Better Health

