Solutions for rapid and sustained patient access to precision medicine (Guest blog)
Healthcare systems are continuously in need for innovative health technologies, such as medicines, in order to improve the quality of life or life expectancy (or both) of patients. Optimal patient access means that all patients who can benefit from these innovations obtain rapid and sustained access to them, when and where they need it, and at a right price. In reality, however, it is observed in the EU that patient access to innovation is still hampered, leading to suboptimal treatment. A recent report on access to oncology therapies in Europe illustrates clearly the patient delay in access issue and the high variability in time to access. [i] According to that report, a key barrier for (early) access to these therapies is related to difficulties in meeting the evidence requirements from Health Technology Assessment (HTA) bodies, as well as lack of consensus between healthcare payers and industry about the interpretation of value for money and affordability of innovations.
Precision medicine is in this regard a promising approach. EFPIA defines precision medicine as “a healthcare approach that utilises molecular information (genomic, transcriptomic, proteomic, metabolomic, etc), phenotypic and health data from patients to generate care insights to prevent or treat human disease resulting in improved health outcomes”. [ii] With the precision approach patients can benefit from a more tailored approach with higher chances for success. This helps to avoid unnecessary adverse events, or the loss of precious time, and can therefore lead to improved overall effectiveness.
It is therefore expected that precision medicine can facilitate patient access to innovations since healthcare payers only need to pay for therapies that have the most likely benefit. [iii]
Challenges with patient access to precision medicine
Yet, several barriers have been identified that hamper patient access to precision medicine. As a result, both access to biomarker testing as well as access to precision-based therapies are inconsistent across Europe and contribute to an imbalance in health equity. These barriers can be grouped within four main categories
A. Barriers related to evidence generation.
Precision medicine is often associated with small patient groups which makes evidence generation more challenging. Therefore, the available evidence at the time of submission for HTA may be limited. Indeed, large Randomized Controlled Trials (RCTs) might not be feasible in all situations, and there is a need to develop alternative designs. Experts have for instance suggested evaluation of disease trends before and after treatment (sometimes referred to as interrupted time series) or running a (small) single‐arm trial and compare the single‐arm trial data with matched external controls, [iv] but HTA bodies may be reluctant to accept such designs. Recently, new trial designs (e.g., basket and umbrella trials) have been proposed for assessing the value of precision medicine, and some HTA bodies actually have provided guidance on their evaluation but it is not clear to what extent these designs are sufficiently convincing for HTA bodies. [v] [vi]
B. Barriers related to the disconnect between the authorisation/reimbursement process of the biomarker and the treatment.
Intrinsically to precision medicine, the choice of therapy depends on the outcome of the applied biomarker test. Due to historically different pathways, both for market authorisation as for price & reimbursement decisions, the “diagnostic” and the “therapeutic” element of a precision approach are often assessed in isolation from each other, thereby applying different assessment criteria, methods and processes. The lack of timely alignment between these two processes can lead to suboptimal situations whereby a medicine is reimbursed by the healthcare system but the corresponding biomarker is not, which can increase inequity in care. [vii]
C. Barriers due to lack of healthcare system readiness
Even when biomarker and therapy are reimbursed, a lack of knowledge about the precision approach, diverse laboratory infrastructures, lack of clinical guidelines and patient reference patterns are observed, hence hampering the appropriate and timely use of precision medicine for the eligible patients. [viii] [ix]
D. Barriers related to the lack of a Pan European high quality and efficient data infrastructure
Data in Europe regarding medicines and biomarkers are scattered, have different formats, quality assurance systems and access facilities both within and between countries. This hampers not only market access but also, in case market access was obtained, the possibility in a real world setting to confirm on a regulatory level the risk/benefit of the precision approach and also to respond to the need of HTA bodies and payers to demonstrate that the precision approach delivers against the promises that were made at launch. [x] Of note, real world data and real world evidence can also play a crucial role during the development process of new medicines in order to better document disease courses during the current standard of care. [xi]
Recommended solutions to improve rapid and sustained patient access to precision medicine
In order to advance the use of precision medicine and tackle the delays and inequalities in patient access, the following recommendations for solutions are formulated. Between brackets, the stakeholders who are invited to take initiative for action are mentioned. Figure 1 provides a schematic overview of the barriers and recommendations.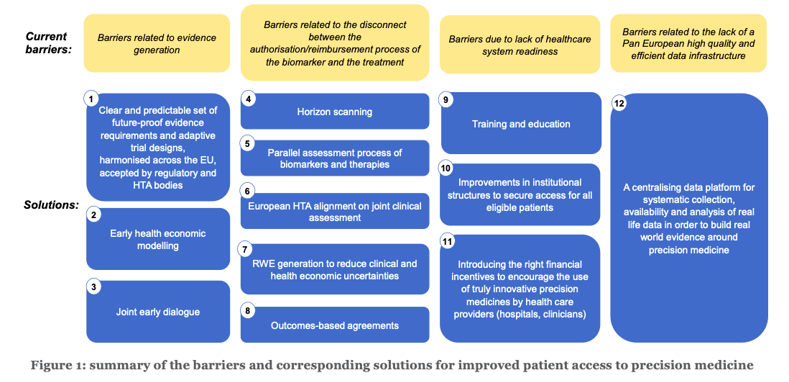
Regarding evidence generation
1. Regulatory bodies and HTA bodies to provide a clear and predictable set of future-proof evidence requirements and accepted designs, adapted towards the specific nature of precision medicine and the smaller target patient populations, thereby ensuring consistency between member states about these requirements [xii] (European Commission, EMA, competent bodies, HTA bodies)2. Companies to anticipate during the early development phases on the eventual value and cost effectiveness of different biomarker-treatment combinations via early health economic modelling. [xiii] Such early models apply a target product profile (TPP) indicating the expected benefits of a health technology (in the case of precision medicine the combination of a diagnostic and a treatment) and estimate – based on this profile – the potential benefits for patients, the healthcare system and society compared to the current or expected standard of care. The advantage of such an approach is that already in the early development stages it becomes clearer how much added value the technology potentially can deliver, whether it is potentially cost-effective and under which circumstances. (Innovative biomarker and medicines industries)
3. Innovative companies (biomarker and therapy), regulators and HTA bodies to organize joint early dialogues, which ideally should take place before the key evidence is collected. Such dialogues can cover evidence generation plans, expected evidence gaps and how it is envisaged to overcome these gaps. [xiv]Evidence gaps may relate to the diagnostic/treatment combination (for instance the sustainability of a treatment effect), the course of the disease (for instance the relationship between an intermediate endpoint and a hard clinical endpoint), the disease epidemiology (incidence and prevalence), or the healthcare ecosystem (e.g. to which extent the target patients will have access to testing). Clinicians and patient representatives should be invited to participate in these dialogues, ensuring that those innovations that deliver the outcomes that matter most to patients, clinicians and society are prioritised. Such dialogues therefore also enable a multi-stakeholder discussion about the evidence generation plan, the planned design and their acceptance, as well as the feasibility of the further outcome registration and data collection in the context of an outcome-based managed entry agreement in a timely way, i.e. from the start of the registration procedure at the EMA. (European Commission, EMA, HTA bodies, Payers)
Regarding the assessment and reimbursement of precision medicine
4. Policy makers to perform horizon scanning for better understanding of the future health budget impact of precision medicines and horizon shaping to establish the therapeutic area of highest need. (EMA, national competent bodies, HTA bodies)5. A parallel process which aligns the assessment, authorisation and reimbursement decisions of biomarkers and therapies in order to facilitate a simultaneous and consistent assessment of the cost-effectiveness of the biomarker-therapy combinations leading to aligned reimbursement decisions. [xv] Ideally, also clinicians and patient representatives should participate in the assessment process, ensuring that the views of all health stakeholders are captured and reflected in decision making. Indeed, clinical expertise and patient expertise are indispensable for making adequate authorisation and reimbursement decisions. (European Commission, competent bodies, HTA bodies, Payers)
6. European HTA alignment on clinical assessment (after which appraisal takes place at the national level) would improve the timelines to patient access. i The benefit of the proposed European joint clinical assessment (JCA) is that it allows scientific convergence in the overall HTA process, thus replacing a multitude of national and regional clinical assessments. [xvi] It avoids fragmentation of the internal market and access distortions, duplication of work and patient access delays. It is key that safeguards included in the Commission proposal ensure that this joint clinical assessment is used at national level guaranteeing that there is no duplication at Member State level. [xvii]
7. In case of remaining uncertainty regarding the evidence, ensure the adequate collection of high-quality real-world data to complement the evidence package once the precision approach is available to patients in a real-world environment. This will require consistency in data collection between member states and multi-country initiatives. This will also require dynamic and adaptive reimbursement processes, that can be inspired by those that have already been worked out for Advanced Therapy Medicinal Products (ATMPs) and Orphan Medicinal Products (OMPs). [xviii] (European Commission, EMA, multi-country HTA body initiatives)
8. Related to the above, allow for an adaptive approach and outcomes-based agreements. [xix] Such outcomes-based agreements can especially be useful in case of significant uncertainties, either related to the diagnostic/treatment combination, the disease and its course or the healthcare ecosystem. The collection of real-world data can then serve to inform the outcomes of the agreement. [xx] (HTA bodies, Payers)
Regarding health system readiness
9. Training and education about precision medicine, thereby also building awareness among clinicians of available options and required referral patterns. [xxi] (EU, member states, HTA bodies, Payers)10. Organisational measures to allow the market penetration of truly innovative precision medicine, e.g. regional centres of excellence, adequate referral patterns and quality assurance schemes. (member states)
11. Introducing the right financial incentives to encourage the use of truly innovative precision medicine by health care providers (hospitals, clinicians). These should better reflect the value of care by allowing the appropriateness of a service for a given patient in a given clinical situation to play a more meaningful role . [xxii]For instance, if a precision approach reduced the probability for hospital readmission, then the financial incentives for hospitals should not reward readmissions but on the contrary penalize these. (member states’ healthcare policy makers)
Regarding the lack of a Pan European high quality and efficient data infrastructure
12. Systematic collection, availability and analysis of real life data in order to build real world evidence around precision medicine. This requires a centralizing data platform maximizing the value for all stakeholders of the data collected through different systems. A future manifestation of precision medicine can include integration of diagnostic and treatment information with emerging artificial intelligence or machine learning platforms. (EU, member states)
Precision medicine has been recognized for many years now as an approach to improve patient outcomes, and healthcare resilience. Yet, still today several barriers exist hampering the market and patient access to precision medicine. This paper summarized the key barriers and proposes concrete solutions for making progress with the ultimate goal to reach access to all eligible patients who can potentially benefit from it.
Funding & review
The discussion paper was funded by the European Federation of Pharmaceutical Industries and Associations – EFPIA – and reviewed by the EFPIA Precision Medicine Working Group.
[i] https://efpia.eu/media/554646/every-day-counts-improving-time-to-patient-access-to-innovative-oncology-therapies-in-europe.pdf
[ii] https://efpia.eu/about-medicines/development-of-medicines/precision-medicine/
[iii] Berm, EJJ; de Looff, M; Wilffert, B; Boersma, C; Annemans, L; Vegter, S; van Boven, JFM; Postma, MJ; Economic Evaluations of Pharmacogenetic and Pharmacogenomic Screening Tests: A Systematic Review. Second Update of the Literature. PLOS ONE Volume: 11(1) JAN 11 2016
[iv] Eichler HG et al. Randomized Controlled Trials Versus Real World Evidence: Neither Magic Nor Myth. CLINICAL PHARMACOLOGY & THERAPEUTICS | VOLUME 109 NUMBER 5 | May 2021 - 1212
[v] Cooper S, Bouvy J C, Baker L, Maignen F, Jonsson P, Clark P et al. How should we assess the clinical and cost effectiveness of histology independent cancer drugs? BMJ 2020; 368 :l6435
[vi] https://www.has-sante.fr/upload/docs/application/pdf/2019-07/doctrine_de_la_commission_de_la_transparence_-_version_anglaise.pdf
[vii] Faulkner E, Annemans L, Garrison L, Helfand M, Holtorf AP, Hornberger J, Hughes D, Li T, Malone D, Payne K, Siebert U, Towse A, Veenstra D, Watkins J; Personalized Medicine Development and Reimbursement Working Group. Challenges in the development and reimbursement of personalized medicine-payer and manufacturer perspectives and implications for health economics and outcomes research: a report of the ISPOR personalized medicine special interest group. Value Health. 2012 Dec;15(8):1162-71.
[viii] Gill J, Fontrier AM, Miracolo A and Kanavos P. Access to Personalised Oncology in Europe. LSE November 2020
[ix] IQNPath. Unlocking the potential of precision medicine in Europe. Feb 2021
[x] Annemans, L., Makady, A. TRUST4RD: tool for reducing uncertainties in the evidence generation for specialised treatments for rare diseases. Orphanet J Rare Dis 15, 127 (2020).
[xi] https://blogs.bmj.com/bmj/2018/03/06/lieven-annemans-we-need-to-reach-a-common-understanding-about-real-world-data/
[xii] ISPOR Personalized Precision Medicine Special Interest Group. Being Precise About Precision Medicine: What Should Value Frameworks Incorporate to Address Precision Medicine? VALUE HEALTH. 2020; 23(5):529–539
[xiii] Annemans L, Genesté B, Jolain B. Early Modelling for Assessing Health and Economic Outcomes of Drug Therapy. Value in Health, Volume 3, Issue 6, 2000, Pages 427-434.
[xiv] Annemans, L., Aymé, S., Le Cam, Y. et al. Recommendations from the European Working Group for Value Assessment and Funding Processes in Rare Diseases (ORPH-VAL). Orphanet J Rare Dis 12, 50 (2017).
[xv] Payne K, Annemans L. Reflections on market access for personalized medicine: recommendations for Europe. Value Health. 2013 Sep-Oct;16(6 Suppl):S32-8
[xvi] https://ec.europa.eu/commission/presscorner/detail/en/ip_21_3142
[xvii] https://www.efpia.eu/news-events/the-efpia-view/statements-press-releases/all-together-no-one-left-behind-delays-and-the-unavailability-of-medicines-harm-patients/
[xviii] Pani L, Becker K. New Models for the Evaluation of Specialized Medicinal Products: Beyond Conventional Health Technology Assessment and Pricing. Clin Drug Investig. 2021 Jun;41(6):529-537
[xix] Eichler, HG., Barker, R., Bedlington, N. et al. The evolution of adaptiveness: balancing speed and evidence. Nat Rev Drug Discov 17, 845–846 (2018).
[xx] Facey, K., Rannanheimo, P., Batchelor, L., Borchardt, M., & De Cock, J. (2020). Real-world evidence to support Payer/HTA decisions about highly innovative technologies in the EU—actions for stakeholders. International Journal of Technology Assessment in Health Care, 36(4), 459-468.
[xxi] https://www.personalizedmedicinecoalition.org/Userfiles/PMC-Corporate/file/PM_and_VAFs.pdf
[xxii] Song Z, Navathe AS, Emanuel EJ, Volpp KG. Incorporating value into physician payment and patient cost sharing. Am J Manag Care. 2018 Mar;24(3):126-128.
