Unlocking the potential of precision oncology (Guest Blog)
The potential of precision oncology is there. To unleash it, we need to move forward together, working on ways to advance regulatory science, to screen and treat, as well as to try new approaches and new payment models.
Recently, I had the pleasure to participate to the Life Sciences – The Era of Personalised Medicine (europa.eu) event organized by Swedish Presidency of the Council of the European Union.
My break out session was on challenges in evidence generation, regulatory evaluation and patient access, organized by the Swedish Medical Products Agency.
We know that Precision Oncology represents a radical paradigm shift delivering value for patients, healthcare systems and societies. Instead of treating cancer based on the location or origin of the tumor in the body, precision treatments specifically target molecular or genomic alterations in the tumor, sometimes even regardless of the location of the tumor itself. For patients, this means not wasting precious time and tissues receiving a treatment which may be ineffective. For health systems, precision oncology offers the opportunity to avoid unnecessary costs or putting unnecessary burden on the shoulders of an already stretched healthcare workforce.
The break out session focused on the difficulties in conducting randomized controlled trials in populations defined by the presence of a molecular alteration; the challenges in generating the evidence required; the consequent limitations with respect to defining what is the appropriate value of the medicinal product.
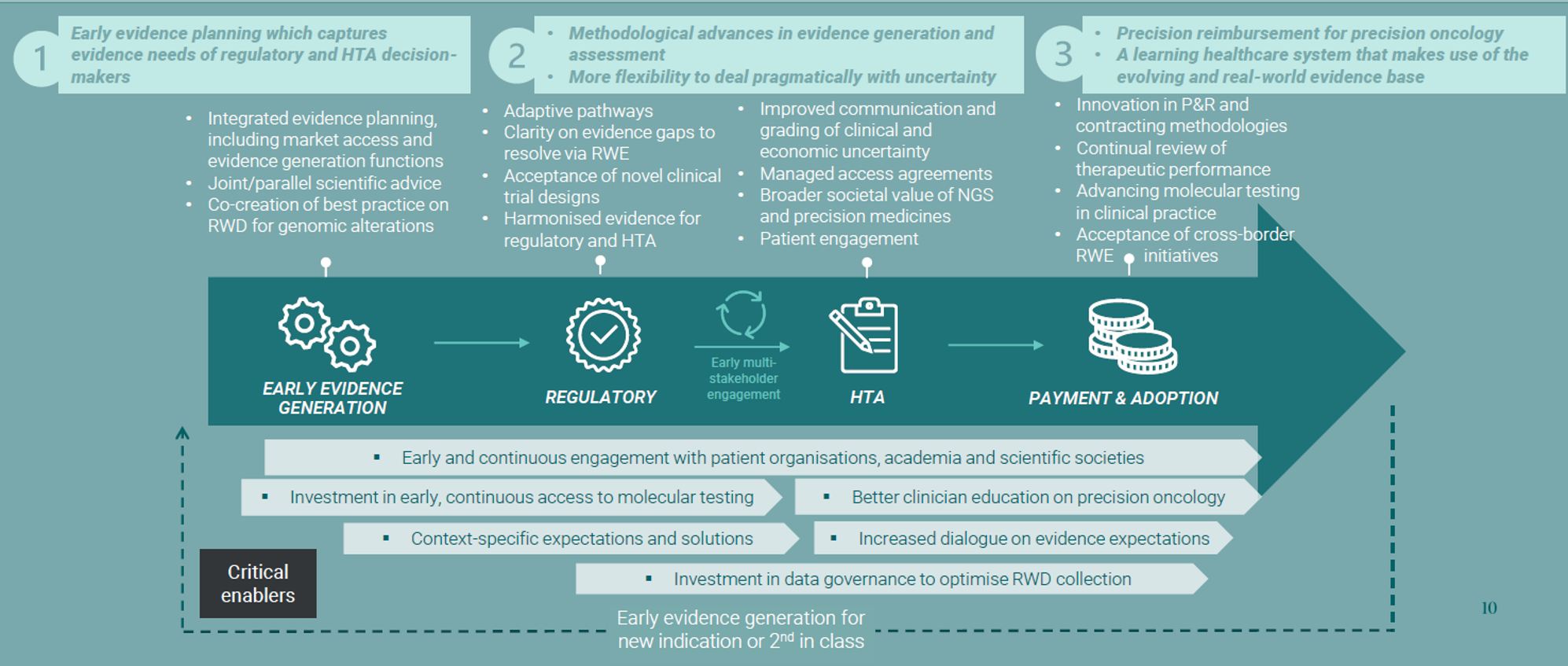
Policies can help addressing these challenges, but we also need an ecosystem shift. To achieve this, we propose to adopt a life cycle approach, with three suggestions:
- Early evidence planning capturing evidence needs of regulator and HTA bodies.
- Methodological advances in evidence generation and assessment coupled with more flexibility to deal PRAGMATICALLY with uncertainty.
- Precision reimbursement for PO, a learning HCS that makes use of evolving and RWE base.
How do we get there? Life cycle approach
Clearing roadblocks to unlock the potential of precision oncology
Think for a moment about the lessons we have learned with the In Vitro Diagnostic Regulaton (IVDR).
Innovators have to comply with two legislations: the CTR (Clinical Trial Regulation) and the IVDR (In Vitro Diagnostic Regulation). Delinkage between the two Regulations delays clinical trials launches in Europe and patients access to these trials (New European legislation designed to protect patients is delaying clinical trials for thousands of people with cancer and rare diseases (efpia.eu).
We need the EU Institutions to ensure a horizontal strategic approach through the implementation of the current various initiatives (General Pharma Legislation, HTA, EHDS, Cancer Plan and Cancer Mission) to avoid such mismatch and de-synchronization.
Each initiative has great potential to improve cancer care in Europe. However, when looking at these initiatives horizontally, are we considering all the interplays and the consequences?
Take the General Pharma Legislation. How will the provisions on trials with comparator arms work with tumor agnostic therapies?
Improving cancer care through broader access to quality biomarker testing
Access to high quality oncology biomarker testing is inconsistent across Europe and contributes to health inequalities both within and between countries. To unlock the potential of precision medicine in Europe (more here) we need to tackle the significant variations in medicines and test access across Europe. How?
By aligning in regulatory & reimbursement approval processes, developing value assessment frameworks for these new technologies and public dedicated budgets, setting up mandatory accreditation and quality assurance schemes for laboratories, promoting centralized testing infrastructures, increasing health literacy, capacity and capability in the system and data collection and sharing.
Finally, while OS remains the most important endpoint, a better recognition of oncology relevant endpoints (OREs) and a value-based approach to pricing can deliver benefits to all.
If this then is coupled with a value-based approach to pricing, we can deliver value to patient, healthcare systems and innovation through novel pricing and payment models, such as outcomes-based managed entry agreements to manage residual uncertainty, and conditional reimbursement linked to further evidence collection.
To realize the potential of precision oncology, we need to deliver harmonized and aligned policies across processes that adapt quickly to the evolving science, empower patients to decide on how to use their data and invest in access to biomarker and biomarker testing.
The potential of precision oncology is there. To unleash it, we need to move forward together, working on ways to advance regulatory science, to screen and treat, as well as to try new approaches and new payment models.
