AI Across the Medicines Lifecycle: Insights from Preliminary Case Studies and Considerations for Policy
As AI becomes more deeply embedded in regulatory decision-making and processes that impact patients, the need for robust governance becomes critical.
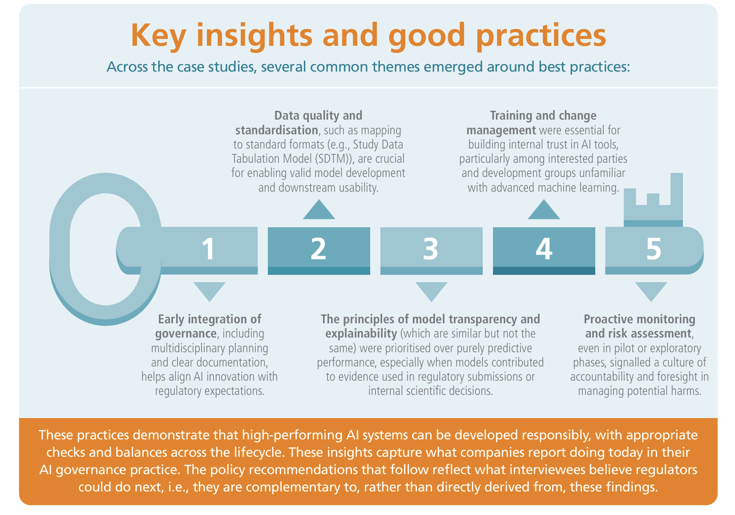
Artificial Intelligence (AI) is transforming the pharmaceutical landscape, offering unprecedented opportunities across the medicines lifecycle, from drug discovery and clinical development to manufacturing and post-approval safety monitoring. As AI becomes more deeply embedded in regulatory decision-making and processes that impact patients, the need for robust governance becomes critical.
This report presents a cross-industry perspective on how AI governance can be integrated effectively into medicinal product research and development (R&D) and post authorisation settings, with a focus on ensuring trust, transparency, and regulatory alignment through ongoing dialogue between industry and regulators.
Read the full report here.