EFPIA Welcomes the European Parliament’s green light to CETA

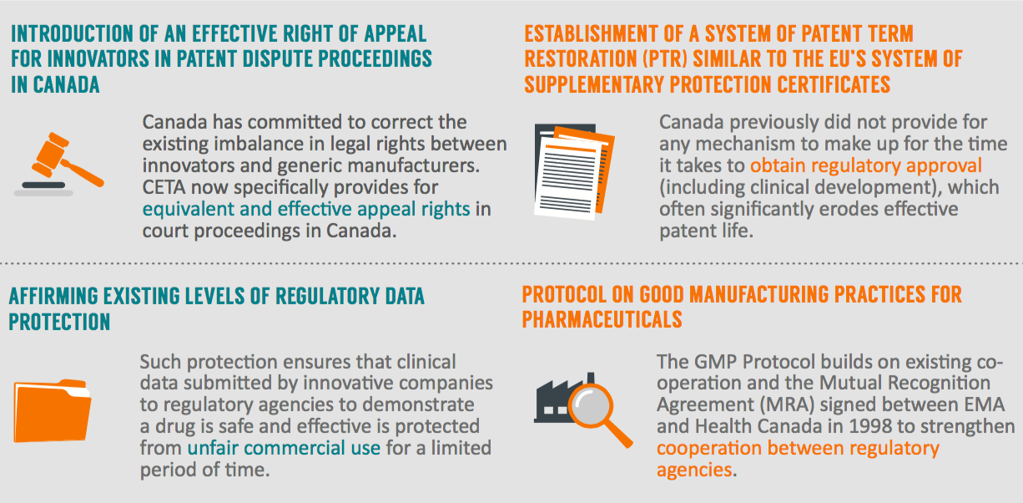
International trade is a key driver of growth, jobs and competitiveness for the research-based pharmaceutical sector in Europe. This underpins our industry’s mission to develop life-saving innovative treatments for patients, which is why the pharmaceutical sector supports the CETA agreement. We believe that the implementation of CETA will represent a positive step forward in levelling the playing field of European pharmaceutical companies trading, investing and researching in Canada.
Furthermore, CETA represents a important opportunity to open new global opportunities for European businesses, both large and small, and sets high standards in negotiating high-quality, cutting-edge free trade agreements between developed countries.
EFPIA Director General Eric Cornut said: “The CETA affords the opportunity for Canada and the EU to move towards a level-playing field in the development of new medicines. We support the agreement and look forward to its swift and effective implementation.”

1) http://trade.ec.europa.eu/doclib/docs/2006/september/tradoc_113363.pdf