Public-private partnerships
IMI is the world’s largest public-private partnership in health
IMI is the world’s largest public-private partnership in health with a total budget of €5 billion – half from the European Union and half from the pharmaceutical industry, through EFPIA. IMI refers to two subsequent programmes: IMI1 under FP7 and IMI2 under Horizon 2020.
IMI carries the torch of medical innovation
EFPIA members are heavily engaged in the Innovative Medicines Initiative (Call 21) to fast track collaborative research, identifying any suitable assets in their libraries that could be utilised to develop diagnostics and treatments in the fight against COVID-19.
The IMI CARE (Corona Accelerated R&D in Europe) project is the largest undertaking of its kind dedicated to discovering and developing urgently needed treatment options for COVID-19. The CARE consortium will accelerate COVID-19 R&D by bringing together the leading expertise and projects of 37 teams from academic and non-profit research institutions and pharmaceutical companies into a comprehensive drug discovery engine.
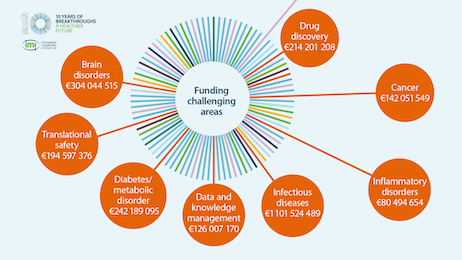
Public-private collaboration delivers to people: some examples
What is the impact of IMI for patients and society?
-
IMI Impact: progress driven by partnership (Guest blog)
The Innovative Medicines Initiative is tackling the biggest health issues of our time through collaborations that make a difference13.04.21Read Article -
IMI Impact: Driving healthcare’s digital future
The Innovative Medicines Initiative is making it easier to share and use health data for research and care16.03.21Read Article -
IMI Impact: Beating cancer – together (Guest blog)
With €190 million invested since 2008, the Innovative Medicines Initiative (IMI) is advancing the fight against cancer04.02.21Read Article -
The IMI Journey of Central Denmark (Guest blog)
How IMI has enabled the connections in an ecosystem encompassing regional, national and EU actors within collaborative research.13.01.21Read Article -
IMI Impact: reinventing regulatory science, together (Guest blog)
Industry and regulators are collaborating to modernise how medicines are developed, making research more relevant to patients’ lives24.11.20Read Article -
IMI impact: improving the lives of people with diabetes
More than €300 million has been invested in IMI projects designed to improve diabetes care and accelerate the search for cures13.11.20Read Article -
IMI Impact: transforming the lives of children (Guest blog)
The Innovative Medicines Initiative is making a difference to children living with paediatric diseases06.11.20Read Article -
IMI Impact: collaboration is key to dementia challenge (Guest blog)
More than 20 Innovative Medicines Initiative projects are helping advance dementia research03.11.20Read Article -
IMI Impact: tackling antimicrobial resistance together
The Innovative Medicines Initiative is delivering fresh hope in the search for new antibiotics10.07.20Read Article -
The Innovative Medicines Initiative: a partnering machine that has transformed the biomedical ecosystem in Europe (Guest blog)
A blog post by Dr Pierre Meulien, Executive Director, Innovative Medicines Initiative02.09.20Read Article
Additional resources
- Industries contribution to IMI projects Your questions answered get_app
- EFPIA Consortium Agreement Template for IMI2 actions get_app
- Infographic: Checks and audits of EFPIA in kind contributions to IMI projects get_app
- Infographic: IMI - From Idea to Project get_app
- Infographic: EU-Industry Contribution to IMI2 get_app
- Blogs
- IMI website
If you have any questions, please contact the Science Policy team at science-policy@efpia.eu